Originally written by Vanessa Pike-Russell and Lisa Loseke updated by Stacy Griffith
Arthropods (e.g., insects and crustaceans) must molt their exoskeletons periodically in order to grow; in this process the inner layers of the old cuticle are digested by a molting fluid secreted by the epidermal cells, the animal emerges from the old covering, and the new cuticle hardens.
The molting process is a central, and nearly continuous, part of a crab’s life. A hermit crab may spend 90% of its time getting ready to molt, molting, or recovering from a molt. There are many dangers to molting including predation, difficulty in movement as muscles have no ridged points of attachment, desiccation, and the risk of an unsuccessful attempt to exit the old exoskeleton. Eighty to ninety percent of arthropod deaths are related to molting.

A hermit crab will shed their exoskeleton when it becomes too snug about their growing body. Hermit crabs cannot go shopping for new skin, they instead shed their exoskeleton and build up the tender tissues with fluids, and with the help of chitin, they develop a hardened exoskeleton. To be able to do this, your hermit crab will need a lot of moisture. You might find your crab near the water dish a lot prior to a molt. If you were to watch your crab molt, you would see your crab stretch and twist until the exoskeleton splits, then slips out of it like a suit. Some crabs cannot do this in one piece, so you may see legs and claws strewn about.
Arthropods molt periodically in order to grow and mature. Triggered by hormones released when its growth reaches the physical limits of its exoskeleton, the molting begins (apolysis) when the cuticle separates from the epidermis due to the secretion of a molting fluid into the exuvial (cast-off skin or cuticle) space. The endocuticle (chitinous inner layer of the cuticle) is then reabsorbed and a new epicuticle (outer, shiny or waxy layer) secreted. Ecdysis is the act of shedding whatever remains of the old cuticle.
“The Y-organ (YO), or molting gland, is the source of steroid hormone production and consequent molt cycle regulation in decapod crustaceans, and is responsive to both external environmental and internal physiological signals1,2,3. Control of molting involves a complex interaction between the eyestalk neurosecretory center, which produces inhibitory neuropeptides, such as molt-inhibiting hormone (MIH) and crustacean hyperglycemic hormone (CHH), and the paired YOs in the anterior cephalothorax2,4,5,6. The YO undergoes transitions in physiological properties at critical stages of the molt cycle. During intermolt, the YO is kept in the basal state by pulsatile releases of MIH to maintain low hemolymph ecdysteroid titers7,8. A reduction in circulating MIH relieves YO repression. The activated YO hypertrophies to increase ecdysteroid synthesis; the hemolymph ecdysteroid titer increases and the animal transitions to the premolt stage1,5. Autotomy, the reflexive loss of a limb due to injury, suspends pre molt for a few weeks, which allows time for a new regenerate to form and grow, and the animal molts with a complete set of walking legs5,9,10. A critical transition occurs at mid-premolt, when the animal becomes committed to molt. The committed YO increases ecdysteroid production further and becomes insensitive to MIH and CHH and a regulatory signal associated with the regenerating limb5,10,11. The animal progresses through to ecdysis without delay.”
The Molt Cycle
There are four phases of the molt cycle: inter molt, pre molt, molt, and post molt. During the inter molt, the exoskeleton is fully formed and the animal accumulates calcium and energy stores. It is the longest phase and constitutes the time between molts.
Pre molt starts when the old exoskeleton begins to separate from the epidermis (skin), and the new exoskeleton begins to form below. Calcium and other nutrient are reabsorbed from the old exoskeleton at this time and stored in the tissue of the crab. This serves the dual purpose of softening the old exoskeleton and recycling the calcium for the new exoskeleton. The muscles in the pinchers and limbs then shrink in anticipation for when they are to be pulled out of the narrow joints of the old exoskeleton during the molt. As separation progresses the crab begins to lose some control of it’s appendages. This is likely part of the reason hermit crabs are under ground so long. They must dig down and form a burrow before the cuticle fully separates. If the crab does not dig down in time they will become increasingly more lethargic, finally appearing limp and dead as the actual molt nears.

Molting occurs as the old exoskeleton cracks and the crab pulls out of it backwards. The entire body is shed, even the gills and antennae. Shedding of the antennae can also cause the crab to lose its equilibrium. In surface molters this may explain why they fall on to their side during shed. The new exoskeleton continues to form and is pale and soft. Bloating with water is responsible for the increase in size after a molt. In the case of land crabs who may not have access to water directly after molting, this water comes either from the shell water (which they carry around with them in their shell), and/or from water accumulated in the blood and water sacs during pre ecdysis. This water pressure is used to stretch the new soft exoskeleton into a larger form. After some rest, the crab eats its old exoskeleton as a source of calcium and other nutrients.

Post molt occurs as the new exoskeleton hardens through the two processes of sclerotization (tanning) and calcification. Sclerotization is the chemical process where proteins form chemical bonds between each other to form a more rigid structure. Calcification is the process of putting calcium into the exoskeleton. Also in this phase the muscles grow back to their natural size and the excess water is lost, leaving room for further growth throughout the intermolt.
Step 1: Apolysis — separation of old exoskeleton from epidermis
Step 2: Secretion of inactive molting fluid by epidermis
Step 3: Production of cuticulin layer for new exoskeleton
Step 4: Activation of molting fluid
Step 5: Digestion and absorption of old endocuticle
Step 6: Epidermis secretes new procuticle
Step 7: Ecdysis — shedding the old exo- and epicuticle
Step 8: Expansion of new integument(covering or investing layer)
Step 9: Tanning — sclerotization(The hardening and darkening processes in the cuticle (involves the epicuticle and exocuticle with a substance called sclerotin) of new exocuticle. Now the chitin and protein are laid down and the exoskeleton will become hardened and shiny after a few weeks.
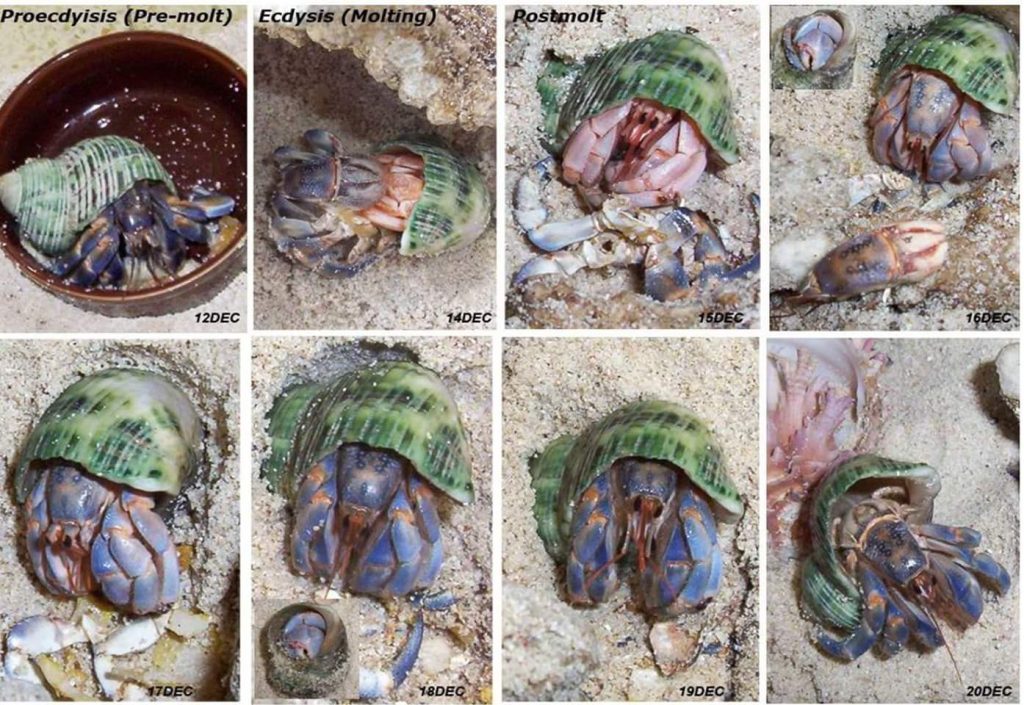
“Typically premolt animals enter their burrows with their abdomens markedly swollen by food reserves… After molting the animal eats its exuviae, which contribute organic materials and calcium salts needed for the new skeleton… Very little information is available in regard to molting of Coenobita. Coenobita clypeatus is reported to hide during the process most of which occurs in the shell (de Wilde, 1973). There is a noticeable reduction in activity for several days prior to the molt and after ecdysis the exuviae are positioned just in front of the mouth of the shell (A.W. Harvey, pers. comm.). During calcification the new soft skeleton of the chelae and other walking legs is molded to fit the shape of the shell. If the animal increases markedly in size it may no longer fit neatly within the old shell and a rapid trade up in shell size may be necessary to avoid water loss and predators. There is no information available on calcium balance or storage through the molt or on growth increments of Coenobita. Coenobita clypeatus grows up to 500 g if large-enough shells are available”
Greenaway, P. 2003 p. 21
Land Hermit Crabs that are eating foods high in calcium, fiber, chitin and foods high in nutrients their bodies need will often have a much higher molting rate; which slows with age or lack of larger seashells. If a crab is in a seashell, which is snug with no alternatives, they will not molt as readily as one with a vast selection.
Exercise is known to increase hunger, and thus will affect the rate of molting. In the wild, land hermit crabs have been known to walk many miles a night, and graze on foods along the way. A hermit crab can be safely exercised in the tank with a plastic hamster wheel.
Scientist Mike Oesterling of the Virginia Institute of Marine Science has noted this in Blue Crabs.
“In the summer months, food availability has a major affect on shedding activity. If a crab does not satisfy the physiological need to shed (increased muscle tissue, body cavity ‘cramping’, etc.), it will not enter the molting cycle. In other words, if it doesn’t get adequate nutrition it’s not going to grow.” Mike Oesterling 2003
Hermit Crabs are social animals, and as such, there is usually a ‘pecking order’ among groups or colonies. As with many animals and organisms, when there is a scarcity of resources you will see a ‘pecking order’ among hermit crabs. The resources most important to hermit crabs being protein and calcium-rich foods and varied diet; hiding spots; space to dig down to molt; different sizes of seashells; water; and salt water.
If a crab is ‘top crab’ than it would get the most food, like with puppies and seagulls. We see this on a small scale within the crabarium, where hermit crabs vie for position in the food bowl or a favourite hiding spot. I have often watched my jumbo hermit crabs fighting for access to the salt-water bowl or Treat dish. It is not unusual for them to fill the bowl completely and keep other hermit crabs away, defending their right to eat first.
The Importance of Water and Lipids
Because water pressure (turgor) is the driving force behind the expansion of the new exoskeleton, it is very important that hermit crabs live in a very humid environment and have access to water that is deep enough to fill their shells. Also, hermit crabs make their blood saltier during a molt to have the water gain necessary for the expansion. Thus a salt water pond is essential for the regulation of this process as well.
What is the role of the pericardial (molt/water) sac?
The pericardial sac plays a significant role in the molting process in terrestrial brachyuran crabs. Prior to molt the sac is filled with water. The water in the pericardial sacs provides turgor pressure to facilitate the lifting of the old exoskeleton.
After the old exoskeleton is shed the water is used to swell the soft body to a new larger size.
Terrestrial crabs use their setae to draw water from damp substrate into the pericardial sac, which are connected to the gill chamber. This indicates the pericardial sac also plays a role in respiration.
Pericardial sac studies thus far have been conducted on species such as Gecarcinus lateralis and Ocypode quadrata but not on Coenobita.
G. lateralis did not fill the pericardial sac from it’s available drinking water. Nor did it update water from substrate that was dampened with sea water. Uptake did not occur at all when only sea water was available. This species only uptakes fresh water via setae to fill it’s pericardial sac.

In the Coenobita family gill structure and size varies between species, particularly between those that live primarily in the forest versus species that are sea water dependent and rarely leave the shore.
“Land Crabs store large quantities of lipids in the hepatopancreas, perhaps representing an adaptation to the variability of terrestrial environments. Unfortunately, few comparative data are available. Charles Darwin (cited in Reyne, 1939) remarked on the fact that over a liter of oil could be rendered from a large B. latro. The hepatopancreas of this animal contains up to 83% lipid (Lawrence, 1970; Storch, Janssen, and Cases, 1982), becoming particularly fat prior to molt (Wiens, 1962). Land crabs may rely heavily on “lipid economy”. Lipid biosynthesis increases markedly prior to ecdysis (O’Conor and Gilbert, 1968) concurrent with the degradation of muscle (particularly the chelae) that permits extracting the limbs through narrow joints in the old exoskeleton (Skinner, 1966b). Subsequent regeneration of muscle, and growth of new muscle tissue, will require nitrogen sources if based on stored lipids” (Wolcott, T. G. 1988. p 90)
Autotomy and Regeneration



“Crabs possess the ability to autotomise their appendages when trying to escape the grip of a predator. The appendages, which detach at preformed breakage planes, are able to regenerate, and require several molts to reach normal size (Weis 1978; Barnes 1986). Because the new cuticle is lost with the autotomised appendage, regeneration only occurs after a complete molting cycle has passed. At this point, the new limb continues to grow beneath the existing but it is doubled over in a folded position (Lee and Weis 1980). At the next molt, the newly generated limb may only appear as a bud or a stump, as it has not had the physical space within which to attain normal size. The new limb continues to grow in a folded position under the hardening exoskeleton until the next molt (Hobbs 1991). This process is repeated until the new limb attains its normal size.” (Charmaine Andrea Huet, 2000)
Molt Care
When your hermit crab digs under and does not resurface for several days you can assume it is molting. A hermit crab that resurfaces each night to eat or drink is not molting. The length of molt depends on size. Micro size hermit crabs can molt in a week. Jumbo sized hermit crabs may take nearly a year. Never dig up a molting crab. One LHCOS member had a hermit crab return after being underground for 14 months.
Hermit crabs do not leave their shell to molt normally. A very, very small percentage may do so, usually because they are missing some limbs and are not able to hold the shell once shedding begins.
Hermit crabs do not always change their shell prior to a molt. Some change after the return from their molt. Some will have a few molts before they change shell, usually because they chose an overly large shell at some point.
Prior to a molt the hermit crab will begin filling up on food and water, filling up their reserves. After the hermit crab finishes shedding their old exoskeleton they will rest for a day or so while their new exoskeleton hardens. The crab will then begin eating their shed exoskeleton for the much needed nutrients it contains. It is not unusual for the hermit crab to leave the big pincer shell and leg tips uneaten.
Some hermit crab owners find that all of their hermit crabs go under to molt at the same. We like to call this phenomenon ‘pet sand’. Once you are certain all the hermit crabs have gone away to molt camp, you can remove the food. As soon as one hermit crab resurfaces begin feeding again. Do not remove your water if it will negatively impact your humidity levels. Continue the normal day/night cycle.
What should you feed post molt? A balanced diet based on our feeding guide, just like any other day. Each daily meal should be at least 50% protein. Only the hermit crab knows what his body needs and if it’s not in the dish he can’t fulfill that need.
NEVER dig up a molter.
No matter how long your hermit crab has been under, do not go digging for it. If the hermit crab has died underground there is nothing you can do about it. The body will naturally decay. If the hermit crab is alive and mid molt you could kill it by digging for it.
Exceptions to the rule: Your tank has flooded and the molters are in danger. You are moving in the next 24 hours and the crab has not surfaced. Your home experienced a fire, strong earthquake or you are under evacuation. Ants have invaded your tank and built a nest.
Digging must be done slowly and carefully, one scoop at a time to protect the hermit crab.
References:
- Bliss, Dorothy E. The Pericardial Sac of Terrestrial Brachyura pages 59-78 in Phylogeny and Evolution of Crustacea Museum of Comparative Biology 1963
- Charmaine Andrea Huet. Spatial Distribution Of Brachyuran Crabs In Sarawak With Emphasis On Fiddler Crabs (Genus UCA) As Biomonitors Of Heavy Metal Pollution. Institute of Biodiversity and Environmental Conservation UNIVERSITY MALAYSIA SARAWAK 2000 http://www.webcastmy.com.my/unimasresearchgateway/thesis/thesis_0062/chap1.htm
- Crustacea Vol. 9: Integument, Pigments, and Hormonal Processes. Academic Press, Inc. New York.
- Dunham, D. W., and S. L. Gilchrist. 1988. Behavior. Pp. 97-138 in Biology of the Land Crabs, W. W. Burggren and B. R. McMahon, eds. Cambridge: Cambridge University Press.
- Fletcher,W.J. and Amos, M. 1994 Stock Assessment of Coconut Crabs. ACIAR Monograph No.29 32p
- Fletcher, W.J., Brown, I.W., Fielder, D.R., and Obed, A. 1991b. molting and growth characteristics. Pp. 35-60 in: Brown,I.W., and Fielder, D.R. (eds), The coconut crab: aspects of Birgus latro biology and ecology in Vanuatu. Canberra, Aciar Monographs 8.
- Fox, S. (2000) Hermit Crabs : A Complete Owner’s Guide. pp. 27. Barrons Books : NY
- Greenaway, P. 2003. Terrestrial adaptations in the Anomura (Crustacea: Decapoda).
- Lemaitre, R., and Tudge, C.C. (eds), Biology of the Anomura. Proceedings of a symposium at the Fifth International Crustacean Congress, Melbourne, Australia, 9-13 July 2001. Memoirs of Museum Victoria 60(1): 13-26.
- Greenaway, P. 1985. Calcium balance and molting in the Crustacea.
- Biological Reviews 60: 425-454. Herreid, C.F. 1969b. Integument permeability of crabs and adaptation.
- Grubb, P. 1971. Ecology of terrestrial decapod crustaceans on Aldabra. Philosophical Transactions of the Royal Society of London B 260: 411-416.
- Held, E.E. 1965. molting behaviour of Birgus latro. Nature (London) 200: 799-800.
- Hobbs, H. H., III. 1991. Decapoda. In Ecology and Classification of North American Freshwater Invertebrates. J. H. Thorp, and A. P. Covich (eds.). Academic Press, New York, NY, p. 823-858.
- Mason, Carol Ann Function of the Pericardial Sacs during the Molt Cycle of the Land Crab Gecarcinus lateralis.
- Oesterling, Mike of the Virginia Institute of Marine Science. Quote relates to blue crabs. URL: http://www.blue-crab.org/fullmoon.htm.
- S. Shyamal, S. Das, A. Guruacharya, D. L. Mykles & D. S. Durica 2018 Transcriptomic analysis of crustacean molting gland (Y-organ) regulation via the mTOR signaling pathway.
- Rao, Krothapalli R. The Pericardial Sacs of Ocypode in Relations to the Conservaton of Water, Molting, and Behavior.
- Ruppert E. E. and Barnes. 1994. Invertebrate Zoology 6th ed. Saunders College Publishing, Philadelphia.
- Ruppert, Fox & Barnes (2004), pp. 523–524
- Stevenson J. R. 1985. Dynamics of the Integument – Integument, Pigments, and Hormonal Processes: Volume 9: Integument, Pigments and Hormonal Processes. (2012). United Kingdom: Elsevier Science.
- Wolcott, T. G. 1988. Ecology. Pp. 55-96 in: Biology of Land Crabs (W. Burggren and B. McMahon, Eds.), Cambridge University Press, New York.